Oxidase Test: Principle, Procedure, Results
The oxidase test is a technique for detecting the presence of the terminal enzyme system in aerobic respiration called cytochrome C oxidase or cytochrome a3. Usually, the family Enterobacteriaceae gives a negative result, whereas Pseudomonas spp, Aeromonas spp, Vibrio spp, and Neisseria spp give a positive result.
Table of Contents
Oxidase Test Principle
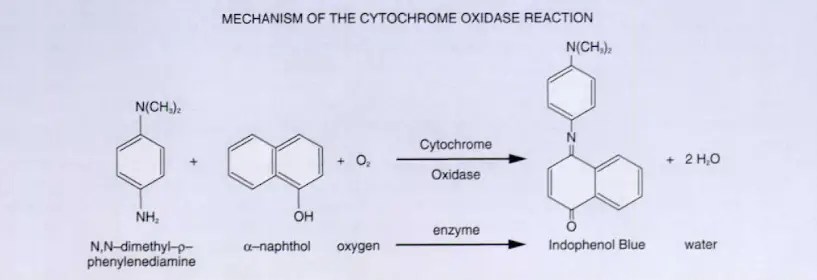
The oxidase test is used to identify bacteria that produce cytochrome c oxidase, an enzyme of the bacterial electron transport chain. When present, the cytochrome c oxidase oxidizes the reagent (tetramethyl-p-phenylenediamine dihydrochloride) to indophenols, a purple or dark blue color end product. When the enzyme is not present, the reagent remains reduced and is colorless.

All bacteria that are oxidase-positive are aerobic and can use oxygen as a terminal electron acceptor in respiration. This does NOT mean that they are strict aerobes. Bacteria that are oxidase-negative may be anaerobic, aerobic, or facultative; the oxidase negative result just means that these organisms do not have the cytochrome c oxidase that oxidizes the test reagent. They may respire using other oxidases in electron transport.
Test requirements for Oxidase test
- Moist filter paper with the substrate (1% tetramethyl-p-phenylenediamine dihydrochloride), or commercially prepared paper disk, wooden wire, or platinum wire.
- Kovács oxidase reagent (1% tetra-methyl-p-phenylenediamine dihydrochloride, in water). Store refrigerated in a dark bottle for no longer than 1 week.

Procedure of Oxidase test
Oxidase test can be performed in various ways. These include, but are not limited to, the filter paper test, filter paper spot test, direct plate method, and test tube method.
Filter Paper Test Method
- Soak a small piece of filter paper in 1% Kovács oxidase reagent and let it dry.
- Use a sterile loop to pick a well-isolated colony from a fresh (18- to 24- hour culture) bacterial plate and rub it onto treated filter paper.
- Observe for color changes.
Note: Nickel, steel and other wire loop give false-positive results, so one should use platinum or inert transfer loop. The alternative options are glass rods, wooden sticks, sterile plastic loops, sterile toothpicks and sterile swabs.

- Oxidase positive: color changes to dark purple within 5 to 10 seconds.
- Delayed oxidase-positive: color changes to purple within 60 to 90 seconds.
- Oxidase negative: color does not change or it takes longer than 2 minutes.
Filter Paper Spot Method
- Use a loop to pick a well-isolated colony from a fresh bacterial plate and rub it onto a small piece of filter paper.
- Place 1 or 2 drops of 1% Kovács oxidase reagent on the organism smear.
- Observe for color changes.

- Oxidase positive: color changes to dark purple within 5 to 10 seconds.
- Delayed oxidase-positive: color changes to purple within 60 to 90 seconds.
- Oxidase negative: color does not change or it takes longer than 2 minutes.
Direct Plate Method
- Grow a fresh culture (18 to 24 hours) of bacteria on nutrient agar or trypticase soy agar using the streak plate method so that well-isolated colonies are present.
- Place 1 or 2 drops of 1% Kovács oxidase reagent on the organisms.
- Do not invert or flood plate.
- Observe for color changes.
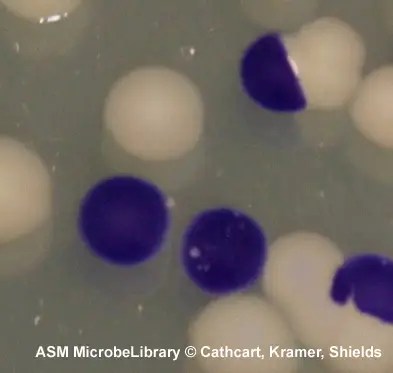
- Oxidase positive: color changes to dark purple within 5 to 10 seconds.
- Delayed oxidase-positive: color changes to purple within 60 to 90 seconds.
- Oxidase negative: color does not change or it takes longer than 2 minutes.
Test Tube Method
- Grow a fresh culture (18 to 24 hours) of bacteria in 4.5 ml of nutrient broth (or standard media that does not contain a high concentration of sugar).
- Add 0.2 ml of 1% α-naphthol, then add 0.3 ml of 1% paminodimethylaniline oxalate (Gaby and Hadley reagents).
- Observe for color changes.

Results
- Oxidase positive: color changes to blue within 15 to 30 seconds.
- Delayed oxidase-positive: color changes to purple within 2 to 3 minutes.
- Oxidase negative: no change in color
Uses of oxidase test
- Oxidase test is most helpful in screening colonies suspected of being a member of the Enterobacteriaceaefamily; all the members of the Enterobacteriaceae family including E. coli are oxidase negative.
- To avoid misidentification, perform an oxidase test on all Gram-negative rods. Oxidase test is especially important in separating Aeromonas from Enterobacteriaceae.
Note: If you see swarming colonies in a culture media, do not perform oxidase test, as its unique characteristics of Proteus spp, which are oxidase negative.
- Oxidase test is used as a major characteristic for the identification of Gram-negative rods that are not in the Enterobacteriaceae family. Colonies suspected of belonging to other genera Aeromonas, Pseudomonas, Neisseria, Campylobacter, and Pasteurella are oxidase positive.
- Gram-negative diplococci give a positive reaction. All members of the genus Neisseria are oxidase positive. Moraxella spp. that are either Gram-negative diplococci or coccobacilli are also oxidase-positive.
Quality Control
Bacterial species showing positive and negative reactions should be run as controls at frequent intervals. The following are suggested:
A. Oxidase positive: Pseudomonas aeruginosa
B. Oxidase negative: Escherichia coli
Precautions and Limitations
- Timing is critical to accurate testing.
- Use fresh reagents, no older than 1 week, older reagents can autooxidize thus giving erroneous results. Do not use if the reagent or filter paper is purple.
- Do not test organisms growing on media that contain glucose or dyes (e.g., MacConkey agar or EMB agar).
- Do not use nickel-base alloy wires containing chromium and iron (nichrome) to pick the colony and make a smear as this may give false-positive results.
- Bacteria grown on media-containing dyes may give aberrant results.
- Older cultures are less metabolically active so may give false-negative results within the mentioned observation time.
The oxidase test must be performed from 5% sheep blood agar or another medium without fermentable sugar. Fermentation of carbohydrates results in acidification of the medium (e.g., lactose in MacConkey Agar or Sucrose in TCBS), and a false negative oxidase test may result if the surrounding pH is below 5.1. Subinoculation on nutrient agar is required before the oxidase test can be performed.
During the identification of suspected Vibrio cholerae isolate, it is not possible to perform an oxidase test directly from a TCBS culture because the acid produced by the sucrose fermenting colonies will inhibit the oxidase reaction.
List of oxidase-positive organisms
Bacterial genera characterized as oxidase-positive include Neisseria and Pseudomonas etc. Genera of the Enterobacteriaceae family are characterized as oxidase negative.
Mneomonics for oxidase positive organisms- PVNCH (It’s just an acronym inspired by the famous mnemonic for urease positive organisms-PUNCH)
- Patricia Shields, Laura Cathcart. 2010. Oxidase test protocol.
- Clinical Microbiology Procedures Handbook, Fourth Edition. (2016). American Society of Microbiology. https://doi.org/10.1128/9781555818814








